ADVERTISEMENT
Capture and Removal of Atheromatous Plaque, From the Lesion Site, Post Directional Atherectomy of Long, In-Stent Restenotic Superficial Femoral Artery Lesions
Download a PDF of this article.
ABSTRACT: The clinical impact of embolic debris released during lower-limb interventions is a source of discussion and controversy. Yet, the sheer magnitude of their number, overall area, and morphology suggest significance, especially in complex and high-risk patients. Technologies designed for lowering embolic burden are required to improve peripheral vascular intervention (PVI) outcomes and to reduce complication rates in a timely and cost-effective manner. This work details capture and removal of atheromatous plaque during post-atherectomy dilatation of long, in-stent, restenotic superficial femoral artery (SFA) lesions in two complex comorbid patients. In both cases, embolic removal was obtained by deployment of an embolic protection device (EPD) prior to the main intervention and by postdilating the lesion using the Proteus aspiration balloon. Captured and removed particles were analyzed and compared for content, count, and dimensions from both the EPD and the Proteus device. Both cases were successfully resolved, as determined by angiography with no sequelae. In both procedures, Proteus surpassed the EPD in the magnitude of removed embolic shower. In case 1, the Proteus balloon captured 228 particles, with a mean major axial dimension of 0.4 ± 0.43 mm (range, 0.12-3.29 mm), while the distally positioned EPD captured 16 particles with a mean major axial dimension of 0.88 ± 1.25 mm. Similarly, 719 particles of a mean 0.24 ± 0.43 mm major axial dimension (range, 0.03-4.83 mm) were recovered in case 2. The capture efficiency presented by the Proteus device over the EPD suggests its potential to serve as a viable tool in complex PVIs, particularly in the dilatation of irregular atherectomized lesions.
J INVASIVE CARDIOL 2013;25(3):E63-E65
Key words: peripheral artery disease, peripheral interventions, distal protection devices, atherectomy
__________________________________________
Peripheral vascular interventions (PVIs) represent a serious and established concern to peripheral circulation by predisposing patients to embolic risks, which can consequentially require reinterventions, such as thrombectomy or thrombolysis.1 Endothelial denudation by means of balloon angioplasty liberates atheromatous plaque in all phases of intervention,2 forming clinically relevant symptomatic emboli at an incidence of 2.4%-30%1,3 and at even higher rates following atherectomy or thrombolytic declotting attempts.1,4-6 The rate of asymptomatic embolization obstructing microvascular beds is believed to be even higher.2,7
While macroemboli are easily detected, and their clinical impact has been firmly established, the clinical relevance of circulating microdebris, particularly in the lower limbs, is becoming increasingly appreciated.2,8 Use of embolic protection devices (EPDs) has become a well-established standard of care in carotid and coronary revascularization procedures, but its routine use in lower-extremity PVIs remains a source of controversy. In addition, contemporary works have presented increasing evidence accentuating the clinical significance of a larger proportion of plaque liberated during PVI than previously believed.1,9 In response, a gradual shift in the protective measures taken during PVI and weight placed on possible side effects is being witnessed. One such measure is the recent Food and Drug Administration clearance of the SpiderFX (ev3) to contain and remove embolic material in conjunction with the TurboHawk atherectomy device for treatment of severely calcified lesions in arteries of the lower extremities.
Emerging safeguarding technologies lowering embolic burden and risk promise to improve PVI outcomes and to reduce their sequelae, particularly among high-risk, multimorbid patients. This report describes two highly complex cases of multimorbid patients presenting with long, severely occluded, in-stent restenotic lesions debulked with atherectomy, followed by Proteus balloon (Angioslide, Ltd) angioplasty for restoration of vessel patency and removal of particles using a suction mechanism. Removed particles were analyzed for content, count, and dimensions. Both interventions concluded with satisfactory angiographic results and no evidence of distal embolization (DE).
Case 1. A 70-year-old female patient with a history of hypertension, diabetes, hyperlipidemia, and cardiovascular disease (CVD) was admitted with severe lifestyle-limiting claudication (RB 3), shortness of breath, and atherosclerosis of the coronary and femoral arteries. Ankle-brachial indexes (ABIs) of the right and left extremities were 0.52 and 0.49, respectively. The right leg was left untreated for this procedure. Angiography of the left leg was performed via a right contralateral arterial femoral approach and revealed a 150-mm long, mildly calcified restenotic and in-stent 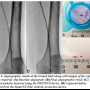 restenotic lesion with chronic total occlusion (CTO) of the superficial femoral artery (SFA) (Figure 1A). Baseline angiography revealed a patent anterior tibial (AT) with reconstitution of the posterior tibial (PT) vessel via collaterals right above the ankle. A 6 Fr crossover sheath was placed in the common femoral artery and the lesion was initially crossed with a 0.35 stiff-angle Glidewire (Terumo Corporation). A 4 mm SpiderFX EPD was deployed distally to the lesion site. Atherectomy was executed using the TurboHawk SxC plaque excision system (ev3). A 4 x 100 mm Proteus embolic capture balloon was used for angioplasty and debris capture and removal within the 4 x 100 mm Supera stent (Idev Technologies, Inc). Post-atherectomy Proteus enabled debris capture and removal upon inward folding and deflation of the balloon, through its suction effect. The rest of the femoral artery was dilated with a 4 x 150 mm NanoCross balloon catheter (ev3). Postprocedural stenosis stood at 10%, and there was neither evidence of embolism during the procedure or within 24 hours of the procedure, nor of clinically significant flow-limiting vessel dissection (Figure 1B).
restenotic lesion with chronic total occlusion (CTO) of the superficial femoral artery (SFA) (Figure 1A). Baseline angiography revealed a patent anterior tibial (AT) with reconstitution of the posterior tibial (PT) vessel via collaterals right above the ankle. A 6 Fr crossover sheath was placed in the common femoral artery and the lesion was initially crossed with a 0.35 stiff-angle Glidewire (Terumo Corporation). A 4 mm SpiderFX EPD was deployed distally to the lesion site. Atherectomy was executed using the TurboHawk SxC plaque excision system (ev3). A 4 x 100 mm Proteus embolic capture balloon was used for angioplasty and debris capture and removal within the 4 x 100 mm Supera stent (Idev Technologies, Inc). Post-atherectomy Proteus enabled debris capture and removal upon inward folding and deflation of the balloon, through its suction effect. The rest of the femoral artery was dilated with a 4 x 150 mm NanoCross balloon catheter (ev3). Postprocedural stenosis stood at 10%, and there was neither evidence of embolism during the procedure or within 24 hours of the procedure, nor of clinically significant flow-limiting vessel dissection (Figure 1B).
Removed particles were flushed, collected in strainer baskets, and stained using violet marking dye (Bradley Products, Inc). Particles were then analyzed using Photoshop CS3 (Version 10.1, Adobe) and Fiji (Image J 1.45b with add-ons). All images were corrected for uneven illumination and then converted to a binary image, where discrete black areas represented embolic particles. Large particles were manually removed from the basket and placed on a uniformly toned surface for imaging. Other particles segregated to the base of the basket. Illumination, threshold, and color channel tools were applied to best suit the imaging conditions of each particle subgroup. Pixel size was converted to metric dimensions (mm) and the Fiji Analyze Particles function was deployed to determine the overall count, and the major axial dimension and surface area of each particle. Analysis of the 228 particles recovered from the Proteus balloon (Figure 1C) demonstrated a mean major axial dimension of 0.4 ± 0.43 mm (range, 0.12-3.29 mm). The distally positioned EPD captured 16 detectable particles (Figure 1D), which featured a mean major axial dimension of 0.88 ± 1.25 mm. Collected particle major axial range was 0.09-4.09 mm.
Case 2. A 67-year-old, obese, hypertensive, hyperlipidemic male presented with debilitating claudication (RB 3) and a right ABI of 0.76. The patient suffered from atherosclerosis of the carotid artery, coronary arteries, and the lower limbs, and had a history of CVD and smoking. A diagnostic angiogram defined a 100-mm long, mildly calcified, 90% stenotic, and in-stent restenotic lesion in  the right SFA (Figure 2A). The lesion was approached in a contralateral fashion and a 7 Fr crossover sheath was placed in the common femoral artery. The lesion was initially crossed with a 0.35˝ stiff-angle Glidewire and a 6 mm SpiderFX EPD was installed to collect any dislodged debris in the popliteal artery. Laser atherectomy was executed using a 2.3 Turbo Elite catheter (Spectranetics). Following lesion debulking, a 5 x 100 mm Proteus balloon was dilated and retrieved after pressure reduction to 2 atm and balloon infolding. Final angiography revealed 3-vessel run-off with no embolisms detected during or within 24 hours of the procedure. No clinically significant flow-limiting vessel dissections were observed and the patient was released with 20% residual stenosis in the treated leg (Figure 2B).
the right SFA (Figure 2A). The lesion was approached in a contralateral fashion and a 7 Fr crossover sheath was placed in the common femoral artery. The lesion was initially crossed with a 0.35˝ stiff-angle Glidewire and a 6 mm SpiderFX EPD was installed to collect any dislodged debris in the popliteal artery. Laser atherectomy was executed using a 2.3 Turbo Elite catheter (Spectranetics). Following lesion debulking, a 5 x 100 mm Proteus balloon was dilated and retrieved after pressure reduction to 2 atm and balloon infolding. Final angiography revealed 3-vessel run-off with no embolisms detected during or within 24 hours of the procedure. No clinically significant flow-limiting vessel dissections were observed and the patient was released with 20% residual stenosis in the treated leg (Figure 2B).
The 719 Proteus-retrieved embolic debris (Figure 2C) were dyed, imaged, and analyzed, as described above, and characterized by a mean major axial dimension of 0.24 ± 0.43 mm. Collected particle major axial range was 0.03-4.83 mm. In contrast, the distally positioned EPD captured 114 particles (Figure 2D), which exhibited a mean major axial dimension of 0.13 ± 0.09 mm. Collected particle major axial range was 0.03-0.57 mm.
Discussion
The frequency of peripheral embolic events, a consequence of PVI, has been grossly underestimated, leaving development and implementation of protection strategies to be further investigated. However, the need for distal protection during endovascular procedures is beginning to attract more attention, especially when treating high-risk patients with comorbidities. Microparticles as small as 0.015-0.1 mm have been proven clinically relevant,10 yet many easily bypass EPDs and require more effective capturing solutions. To date, standard EPDs are reported to prevent circulation of particles with a major axial dimension of 0.05 mm or more, yet are accompanied with clinical and financial limitations and are therefore sparingly used in the lower limbs. As shown here, the Proteus balloon offers a robust and cost-effective embolic capture and removal angioplasty platform for lower-limb indications.
Lesion length, vessel diameter, degree of stenosis, and the nature of the endovascular procedure positively correlate with embolization rates and may be useful in defining embolic profiles requiring tighter anti-embolic regulation during PVIs.9 In the presented cases, the restenotic nature and significant length and degree of occlusion of the lesions categorized them as cases with significant risk of DE.1,11,12 Our present experience with the Proteus device validates the efficacy of embolic capture angioplasty and stands in accord with previously reported applications in similar interventions.13-16 Angioplasty performed immediately post-atherectomy removed particles of over 2 mm in diameter in both cases and a high volume of smaller particles, under 0.5 mm in diameter (818 particles in total). While no histopathological assessment was conducted in this study, particle consistency, in our experience, suggests evidence of atheromatous plaque debris that most likely detached from the vessel wall during the angioplasty phase of the atherectomized lesion.
Recent bench test experiments conducted by Siewiorek et al17 may help shed some light on the large variations between the capture volumes of PROTEUS and the SpiderFX EPD. In the article mentioned, the capture efficiency of some of the EPD devices tested (SpiderFX, Angioguard RX [Cordis Corporation], and Accunet [Abbott Vascular]), ranged from 1.5%-31.79% with particles of under 150 μm and 19.34%-79.71% for 200 μm particulates. Commonly, particles of that magnitude tend to be disregarded due to their “clinically irrelevant” size. However, in current settings, the sheer magnitude of their number, overall area, and morphology suggest that further investigation of their clinical impact on patency, perfusion, and reintervention rate is required, particularly when considering the scale of distal vessel diameters.
DE is a poorly reported phenomenon, with confusing and inconsistent literature. A 2011 review by Wholey18 reported that disclosure of incidence ranges from 1.6%-100%, with the highest number of DE events reported in atherectomy, mechanical thrombectomy, thrombolysis, long, thrombotic and/or total occlusions. Shrikhande et al (2011)11 and Shammas et al (2012)19 reported that DE requiring further treatment occurs in 2%-3% of unselected infra-inguinal patients and is largely patient-, lesion-, and procedure-dependent, with significant variations between groups. Davies et al (2010)20 reported in their analysis that patients suffering from critical limb ischemia, no/poor flow, or tissue loss, or patients presenting at baseline with occlusions and TASC II D lesions, are at greater risk of DE events. Davies also reported that 26.5% of the DE group of patients suffered from deterioration in symptoms following endovascular intervention. One major data limitation is that to date, there has been no prospective study or registry conducted with DE, its consequences, and/or the efficacy of DE preventative action as a primary endpoint in lower-limb settings.
Considering the above, until such data are available, it is up to the primary operator to calculate the risk/cost patient benefit of when and how embolic reduction strategies should be implemented. It is important to note that despite current findings, it cannot be claimed that all embolization events can be avoided using the embolic reduction strategies. The conclusions drawn here are limited to the presented two cases, for which no histopathological assessment was conducted. All points mentioned will require further investigation in a larger cohort study.
References
- Shammas NW, Dippel EJ, Coiner D, et al. Preventing lower extremity distal embolization using embolic filter protection: results of the PROTECT registry. J Endovasc Ther. 2008;15(3):270-276.
- Lam RC, Shah S, Faries PL, et al. Incidence and clinical significance of distal embolization during percutaneous interventions involving the superficial femoral artery. J Vasc Surg. 2007;46(6):1155-1159.
- Shammas NW, Shammas GA, Dippel EJ, et al. Predictors of distal embolization in peripheral percutaneous interventions: a report from a large peripheral vascular registry. J Invasive Cardiol. 2009;21(12):628-631.
- Kaid KA, Gopinathapillai R, Qian F, et al. Analysis of particulate debris after superficial femoral artery atherectomy. J Invasive Cardiol. 2009;21(1):7-10.
- Chalmers RT, Hoballah JJ, Kresowik TF, et al. Late results of a prospective study of direct intra-arterial urokinase infusion for peripheral arterial and bypass graft occlusions. Cardiovasc Surg. 1995;3(3):293-297.
- Wholey MH, Maynar MA, Pulido-Duque JM, et al. Comparison of thrombolytic therapy of lower-extremity acute, subacute, and chronic arterial occlusions. Cathet Cardiovasc Diagn. 1998;44(2):159-169.
- Thompson MM, Smith J, Naylor AR, et al. Microembolization during endovascular and conventional aneurysm repair. J Vasc Surg. 1997;25(1):179-186.
- Al-Hamali S, Baskerville P, Fraser S, et al. Detection of distal emboli in patients with peripheral arterial stenosis before and after iliac angioplasty: a prospective study. J Vasc Surg. 1999;29(2):345-351.
- Karnabatidis D, Katsanos K, Kagadis GC, et al. Distal embolism during percutaneous revascularization of infra-aortic arterial occlusive disease: an underestimated phenomenon. J Endovasc Ther. 2006;13(3):269-280.
- Hori M, Gotoh K, Kitakaze M, et al. Role of oxygen-derived free radicals in myocardial edema and ischemia in coronary microvascular embolization. Circulation. 1991;84(2):828-840.
- Shrikhande GV, Khan SZ, Hussain HG, at el. Lesion types and device characteristics that predict distal embolization during percutaneous lower extremity interventions. J Vasc Surg. 2011;53(2):347-352.
- Kearney M, Pieczek A, Haley L, at el. Histopathology of in-stent restenosis in patients with peripheral artery disease. Circulation. 1997;95(8):1998-2002.
- Banerjee S, Iqbal A, Sun S, at el. Peripheral embolic events during endovascular treatment of infra-inguinal chronic total occlusion. Cardiovasc Revasc Med. 2011;12(2):134.e7-e10.
- Zankar A, Brilakis E, Banerjee S. Use of embolic capture angioplasty for the treatment of occluded superficial femoral artery segments. J Invasive Cardiol. 2011;23(11):480-484.
- Zankar A, Brilakis ES, Banerjee S. Embolic capture angioplasty of lower extremity lesion following distal embolization. Cardiovasc Revasc Med. 2011;12(5):337-340.
- Hadidi OF, Mohammad A, Zankar A, et al. Embolic capture angioplasty in peripheral artery interventions. J Endovasc Ther. 2012;19(5):611-616.
- Siewiorek GM, Wholey MH, Finol EA. A comparative analysis of bench-top performance assessment of distal protection filters in transient flow conditions. J Endovasc Ther. 2012;19(2):249-260.
- Wholey M. The role of embolic protection in peripheral arterial atherectomy. Tech Vasc Interv Radiol. 2011;14(2):65-74.
- Shammas NW. Balloon angioplasty with built-in embolic protection mechanism: the dual role of the proteus balloon. J Endovasc Ther. 2012;19(5):617-619.
- Davies MG, Bismuth J, Saad WE, at el. Implications of in situ thrombosis and distal embolization during superficial femoral artery endoluminal intervention. Ann Vasc Surg. 2010;24(1):14-22.
__________________________________________
From the Director of Cardiovascular Research, Community Health Care System Munster, Indiana.
Disclosure: The author has completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Makam reports that he is a paid consultant, investor and is on the Speaker’s Bureau for Cardiovascular Systems, Inc. He also trained for Angioslide Ltd in 2012.
Manuscript submitted August 6, 2012, provisional acceptance given August 27, 2012, final version accepted November 19, 2012
Address for correspondence: Prakash Makam, MD, 10010 Donald Powers Drive Munster, IN 46321. Email: prakashmakam@comcast.net











